What Is The Final Temperature Of The Methane Once The System Equilibrates?
What is the final temperature of the methane once the system equilibrates?. The normal boiling point of methane is -1615 C. The specific heats of liquid and gaseous methane are 348 and 222 JgK respectively. Yeah it would be 1115 Calvin.
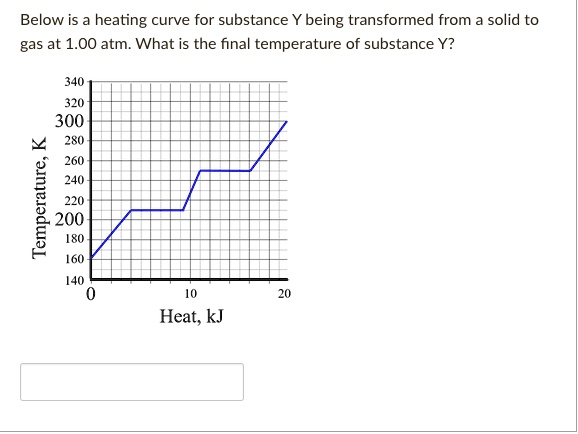
Heat of vaporization is 820 kJmol. The normal boiling point of methane is -1615C. Gaseous What is the final temperature of the methane once the system equilibrates.
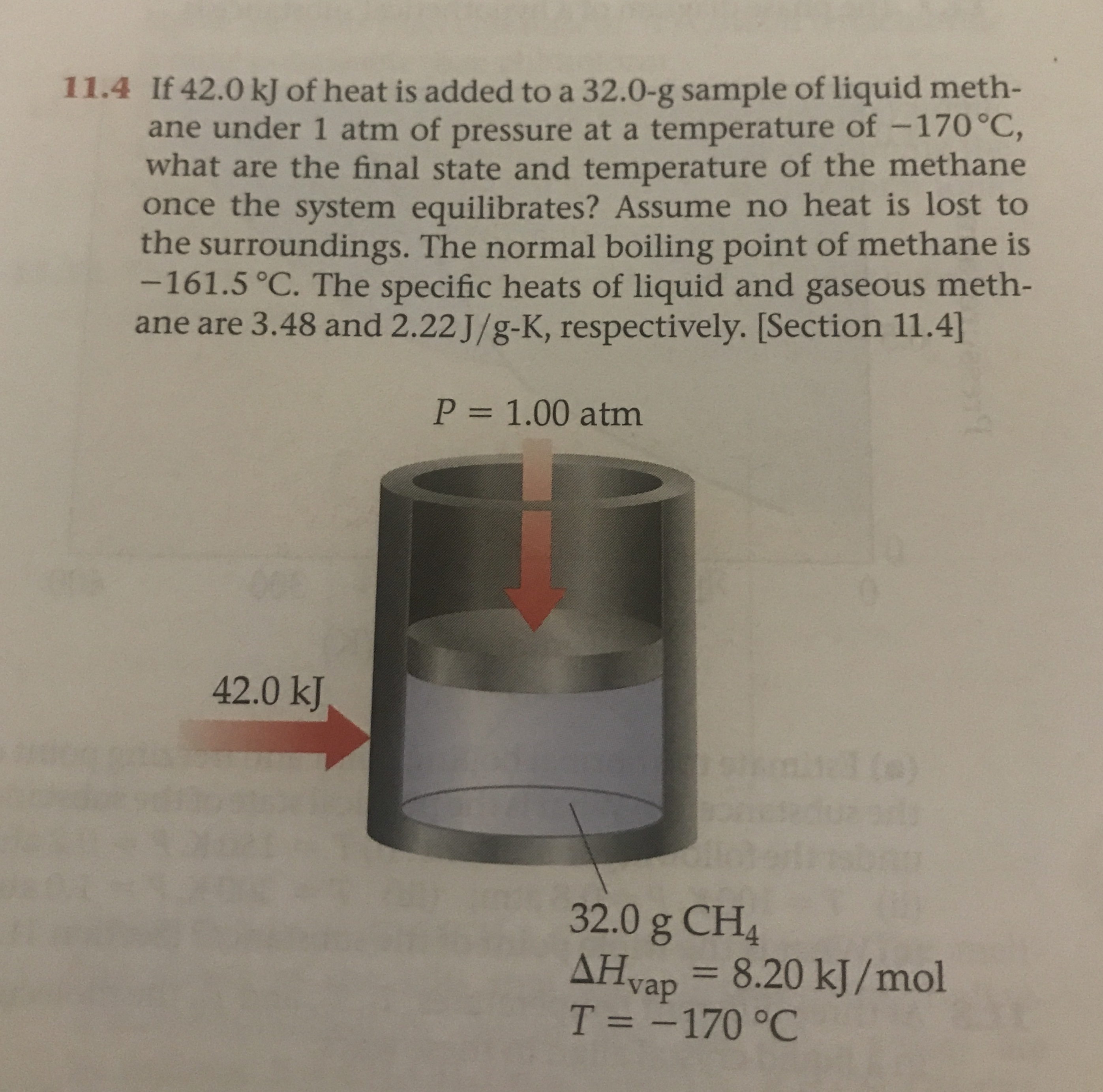
The specific heats of liquid and gaseous methane are 348 and 222 JgK respectively. Assume no heat is lost to the surroundings. If 420 kJ of heat is added to a 320 g sample of liquid methane under 1 atm of pressure at a temperature of -170C what are the final state and temperature of the methane once the system equilibrates.
See we have you 470 all jewels over 32 times two points to two. Assume no heat is lost to the surround-ings. If 420 kj of heat is added to a 320-g sample of liquid methane under 1 atm of pressure at a temperature of 170c what is the final state of the methane once the system equilibrates.
The normal boiling point of methane is 1615 c. 3 on a question. So minus 170 degrees Celsius would be and Calvin.
The specific heats of liquid and gaseous methane are 348 and 222 J g. So first of all the heat required. The normal boiling point of methane is 1615 c.
The normal boiling point of methane is -1615 C. If 420 kJ of heat is added to a 320-g sample of liquid methane under 1 atm of pressure at a temperature of -170 C what are the.
So first of all the heat required.
Assume no heat is lost to the surround-ings. From minus 170 degrees Celsius tu minus 1615 degrees Celsius. Assume no heat is lost to the surroundings. The normal boiling point of methane is -1615 C. The specific heats of liquid and gaseous methane are 348 and 222 Jg-K respectively. If 420 kJ of heat is added to a 320-g sample of liquid methane under 1 atm of pressure at a temperature of -170 C what is the final state of the methane once the system equilibrates. Gaseous What is the final temperature of the methane once the system equilibrates. Could be the final temperature. Assume no heat is lost to the surroundings.
If 420 kj of heat is added to a 320-g sample of liquid methane under 1 atm of pressure at a temperature of 170c what is the final state of the methane once the system equilibrates. Assume no heat is lost to the surroundings. Start your trial now. Section 114 P 100 atm. See we have you 470 all jewels over 32 times two points to two. Assume no heat is lost to the surround-ings. Gaseous What is the final temperature of the methane once the system equilibrates.










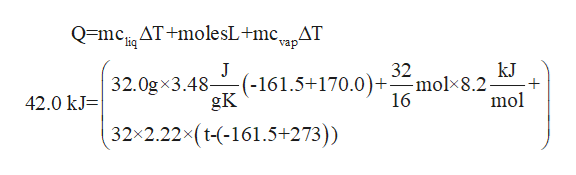






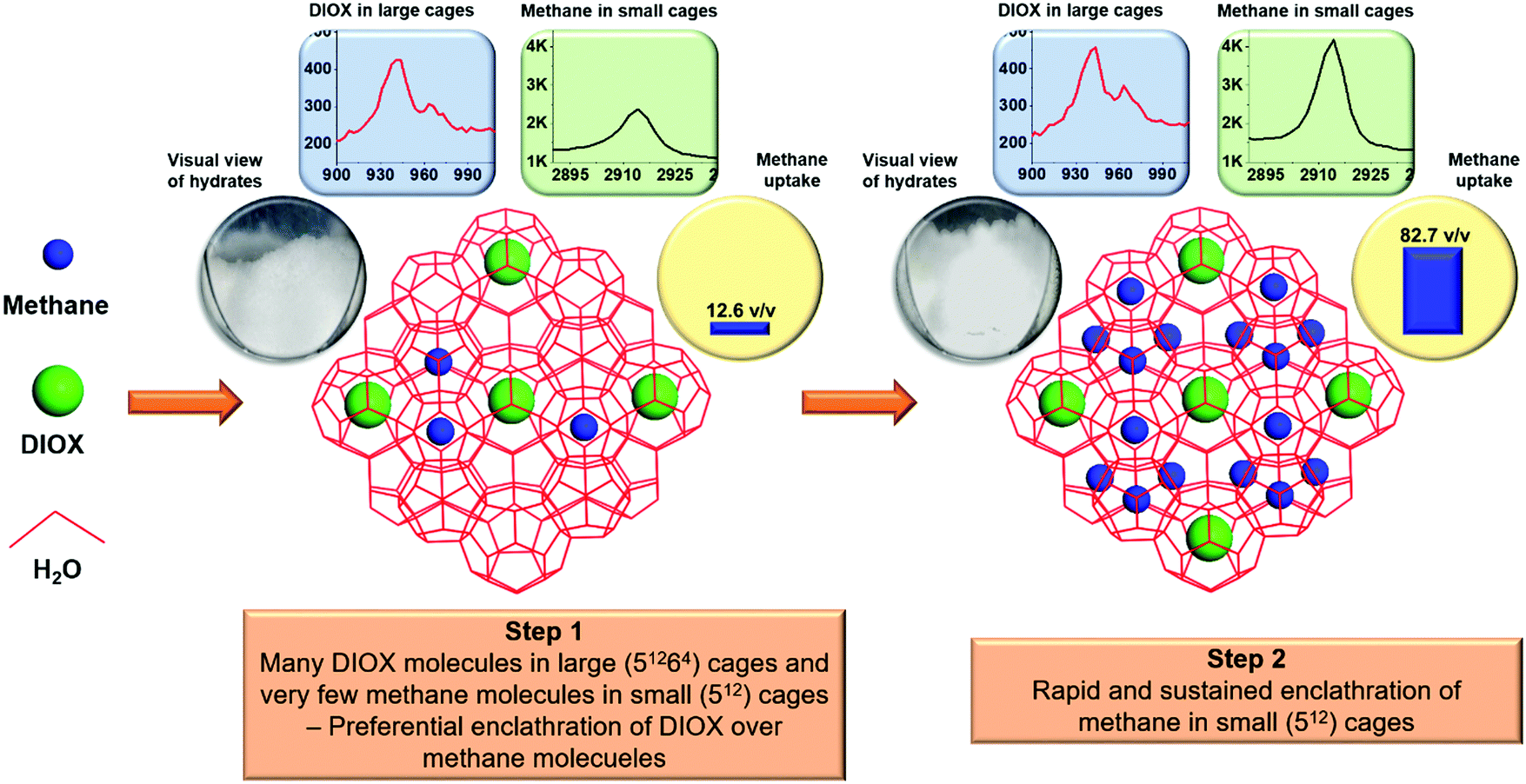
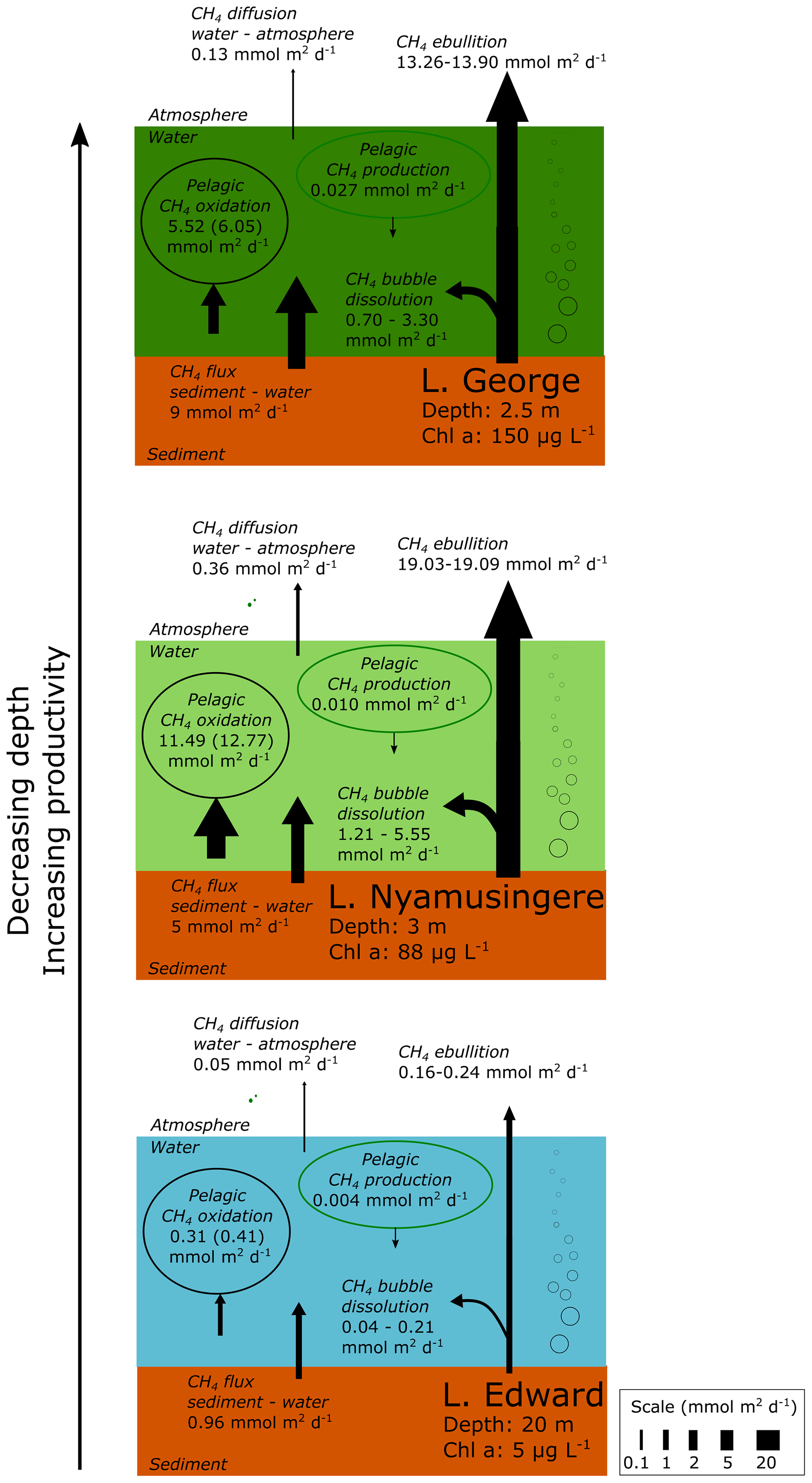


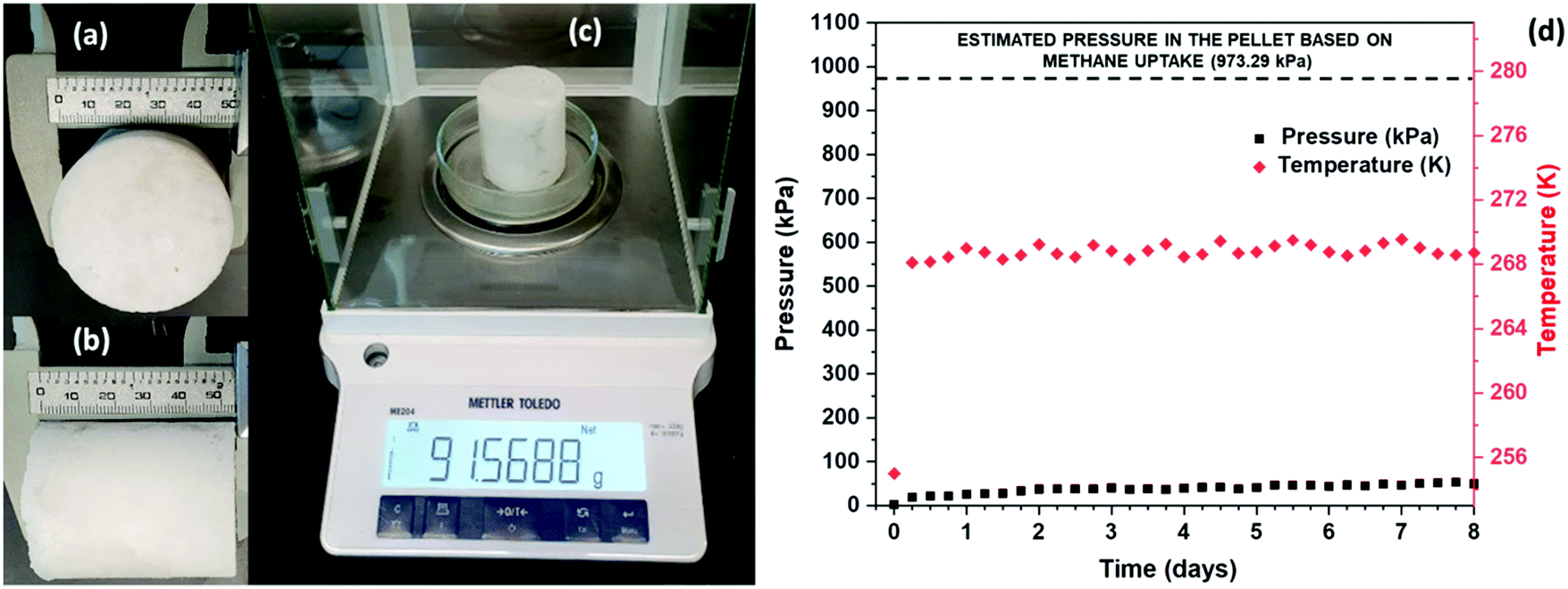

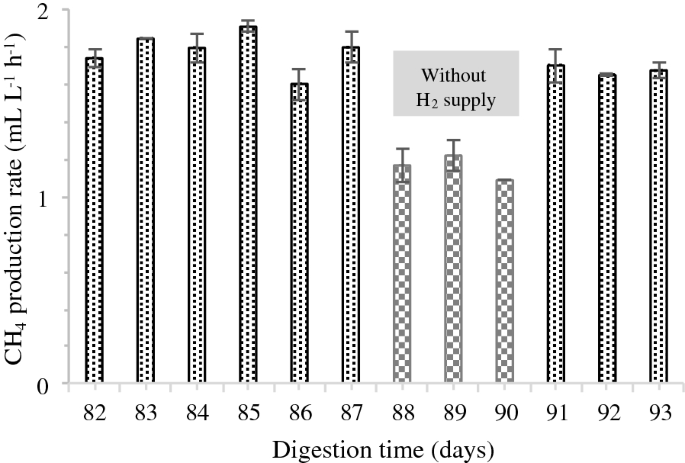


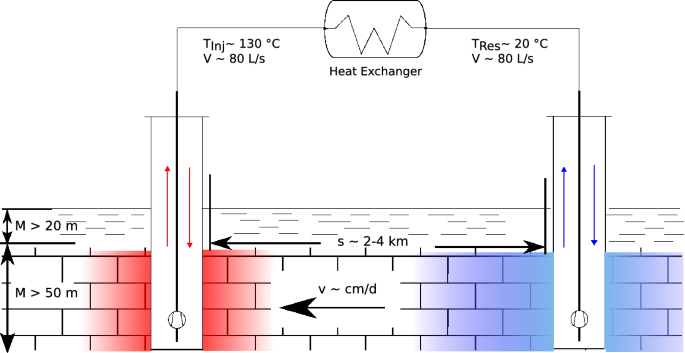





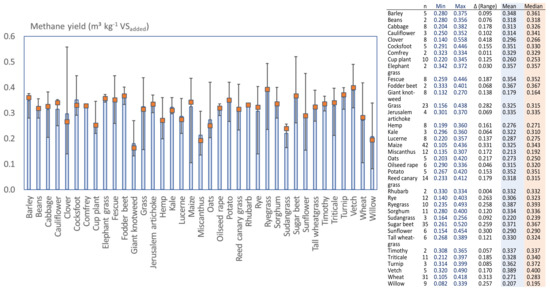
Post a Comment for "What Is The Final Temperature Of The Methane Once The System Equilibrates?"